Abstract
Background
Sickle cell disease (SCD) is an inherited red blood cell disorder which leads to significant morbidity and early mortality. The most widely available curative approach remains hematopoietic stem cell transplantation (HSCT). However, <15% SCD patients have human leukocyte antigen (HLA)- matched sibling donors. HLA-haploidentical (haplo) HSCT expands the donor pool considerably and is a practical alternative for these patients, but with an increased risk of allograft rejection. Biomarkers in patient plasma could potentially be helpful in predicting HSCT outcome and allow treatment at an early stage so that graft rejection could be reversed or prevented.
Objective
The purpose of the study was to detect variations in the plasma proteomes of engrafted patients as compared to those who rejected their grafts and elucidate mechanisms associated with engraftment.
Methods
Patients were conditioned with alemtuzumab, 400cGy total body irradiation, and a dose escalation of post-transplant cyclophosphamide ranging from 0-100mg/kg (Fitzhugh CD et al. Blood Advances, 2017). Tandem Mass Tag (isobaric tags for relative quantification) was performed on plasma samples from engrafted (N=9) and rejected patients (N=10) who underwent haplo HSCT. Proteins with p-values <0.05, relative difference in protein concentration between groups >25%, and total number identified peptide sequences >2 were considered statistically significant. Bioinformatics tools (DAVID, GeneMANIA, STRING and LENS) were used to analyze the identified proteins. Biologically relevant proteins were further evaluated by ELISA to verify their association with the allograft status.
Results
The proteomics analysis revealed 44 differentially expressed proteins between engrafted and rejected groups. Seven candidate proteins, namely thrombospondin-1 (TSP-1), platelet factor 4 (PF4), talin-1, moesin, cell division control protein 42 homolog, galectin-1 (GAL-1), and CD9 were selected for further analysis. Pathway enrichment analysis revealed that the differentially expressed proteins were mostly related to focal adhesion, rap1 signaling pathway, regulation of actin cytoskeleton platelet activation, and leukocyte transendothelial migration. The interaction network of the identified proteins indicated that the proteins were partially biologically connected.
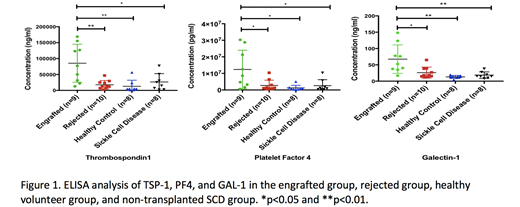
ELISA results of the 7 proteins revealed that TSP-1, PF4, and GAL-1 were significantly higher in the engrafted group as compared with the rejected group, African-American healthy volunteer group, and SCD group who had not been transplanted (see Figure 1). TSP-1 has been found to promote the survival of corneal transplants and is a major activator of transforming growth factor (TGF)-b signaling pathway (Saban DR et al. J Immunol, 2010), an important player in the initiation of immunological tolerance. PF4 has been observed to be associated with improved outcome in cardiac and kidney transplants (Shi G et al. J Clin Invest, 2014 and Zhang L et al. Inflammation, 2015), to enhance proliferation of regulatory T-cells, and to decrease Th17 differentiation (Shi G et al. J Clin Invest, 2014). Lastly, GAL-1 has been found to induce regulatory T- and regulatory B-cell function (Alhabbab R et al. Sci Rep, 2018).
Conclusions
We have shown for the first time that TSP-1, PF4,and GAL-1 are associated with engraftment in patients with SCD who undergo haplo HSCT. The study warrants further validation in a larger sample size as well as in vitro and in vivo analyses to evaluate whether our candidate proteins are associated with tolerance induction.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal